Bioenergetics and Dynamics of Mitochondria (BioDynaMit)
The BioDynaMit team gathers great levels of expertise focused on mitochondria (cell biology, genetics and bioenergetics). We aim to elucidate molecular defects underpinning mitochondrial and metabolic disorders to open new diagnostic and therapeutic avenues.

Team Leader : Arnaud Mourier
Dr. Arnaud Mourier’s research is directed at understanding, by using integrative biochemical and functional genomic approaches, how mitochondrial dynamics and activity are regulated and controlled, and how their aberrant morphology or dysfunction contributes to the pathogenesis of complex metabolic disorders, neurodegeneration, myopathy and ageing.
Team Members
Rose Bernadet – AI ANR
Chloe Barsa – PhD student
Claudine David – IEHC CNRS
Guillaume Duranthon – IE ANR
Camille Evain – PhD student
Orane Lerouley – PhD student
Arnaud Mourier – CRCN CNRS
Philippe Pasdois – MCU
Manuel Rojo – DR2 CNRS
Former Team Members
PhD Students : Thibaut Molinié – Giovana Rech (visiting) – Claire Larrieu – Maya Moubarak
Postdoc : Cyrielle Bouchez – Boutaina Daher – Celia Fernandez-Sanz – Irati Romero – Gro Vatne Rosland – Diwas Srivastava (visiting postdoc) – Antonio Pagano Zottola
ITA : Elodie Cougouilles – Sylvain Cuvellier
Master Students: Narda Arda – Jordan Cougouilles – Celya Duparc – Guillaume Duranthon – Ferdinand Got – Esra Karatas – Florian Magendie – Thibaut Molinié – Julian Perrin – Preslia Okou – Anaïs Rey – Sarah Sayegh.
Researchers/MCU: Thomas Daubon – Océane Martin
Main Research Projects
Mitochondria are double membrane organelles, which hold a central role in cell metabolism as they couple the oxygen consumption to the production of the energy currency ATP through the oxidative phosphorylation (OXPHOS) system. Due to their key energetic role, the localization of mitochondria at intracellular sites of high-energy demand is crucial to maintain cell energy metabolism. In muscle, mitochondria are embedded between myofibrils that consume ATP during contraction. Likewise, in neurons, mitochondria are transported and accumulate in synapses to provide the energy required to maintain and regulate neurotransmission. Their intracellular distribution depends on their mobility and their highly plastic morphology, which results from balanced fusion and fission processes. Beyond its role in maintaining proper mitochondrial morphology and subcellular location, mitochondrial dynamics is key to maintain mitochondrial genome, which is essential to ensure proper OXPHOS assembly and activity. During the past two decades, a significant amount of relevant data has been obtained on the proteins involved in mitochondrial fusion/fission dynamics, notably several dynamin related proteins (DRPs: DRP1, OPA1, MFN1, MFN2). Interestingly, pathogenic mutations in OPA1 and MFN2 cause neuropathies ranging from mild to severe form, causing dominant optic atrophy or peripheral neuropathies as Charcot-Marie-Tooth (CMT) type 2A disease. The pathological spectrum associated with disturbed mitochondrial dynamics has recently expanded to include Parkinson’s, Huntington’s and Alzheimer’s diseases. Pathogenic mutations involved in Parkinson’s and Huntington’s diseases have been associated with an excessive mitochondrial fragmentation. The main goals of our research is to elucidate pathogenic mechanisms underpining the CMT2A neuropathy and more specifically: to understand the isoform selectivity (no MFN1 mutations identified) and the tissue-specificity of MFN2 associated diseases.
As they transduce redox energy into ATP (the cell chemical energy currency), mitochondria are commonly depicted as cellular energy powerplant. Our research has recently demonstrated that, beyond its role in ATP production the mitochondrial respiration play a key role in orchestrating cell metabolism. The fact that the respiratory chain, beyond energy production could orchestrate its energy fuelling acting as a metabolic conductor is a true paradigm shift in Biology. The aim of BioDYnaMit research is to elucidate regulatory mechanisms orchestrating mitochondrial energy fuelling and activity to fit tissue specific needs. The overarching goal of our research is to better understand the great disparity in tissue-specific dysfunctions observed in mitochondrial genetic diseases.
Research Activity
Scientific goals of the BioDynaMit group
- To unravel molecular mechanism mediating mitochondrial fusion and fission, and developing tools allowing in vivo quantification of these processes.
- To decipher the link between mitochondrial morphology, mitochondrial genetics and bioenergetics.
- To investigate modulation of OXPHOS system structure and composition allowing mitochondria to adapt to various tissues specific energy demands under physiological or pathological conditions.
- To elucidate physiopathological mechanisms underpinning mitochondrial dynamics-related diseases
Skills and expertise
Mitochondrial dynamics: We have several tools allowing visualising and characterizing mitochondrial morphology and evaluating mitochondrial dynamic activity. We also develop new tools to investigate inter-organelar communication.
Mitochondrial bioenergetics: The lab is equipped with last generation Oroboros oxygraph O2K instruments, fluorimeter and spectrophotometer allowing proper and accurate characterization of cellular and mitochondrial bioenergetics properties. To this end we assess oxygen consumption of cells, permeabilized cells or isolated mitochondria; mitochondrial membrane potential, ATP production, ROS production rate and enzyme activity and content of OXPHOS system. We also characterize the supramolecular organization of the OXPHOS system by BN-PAGE (Blue Native Polyacrylamide Gels)
Active Collaborations
National
- The BioDynaMit laboratory is collaborating with Dr. Pierre Bon (XLIM, CNRS 7252) – Prof. Agnès Nadjar (INRA-UMR1286) – Dr. Fabienne Foufelle (INSERM U1138) – Dr. Bianca Habermann (IBDM-UMR7288) – Dr. Frédéric Bringaud (CNRS-UMR5234) – Prof. Jean-Charles Portais (Inserm U1031)
International
- BioDynaMit is affiliated with the Department of Medical Biochemical and Biophysics of the Karolinska Institutet and collaborates with Nils-Göran Larsson Group.
- Erika Fernandez-Vizzara (Padoua University UNIPD) – AFM grant 24962
- Chan Bae Park PhD, Ajou University School of Medicine (South Korea), JB. Stewart and D. Milenkovic (MPI for Biology of Ageing, Germany)
National Network
- Le Groupe Français de Bioénergétique (GFB) – http://www.ibpc.fr/UMR7141/GFBioenergetique/
- Meetochondrie – http://www.meetochondrie.fr/meetochondrie/
Keywords
Energy metabolism – Bioenergetics – Mitochondrial dynamics – MFN1 – MFN2 – CMT2A
Complete Bibliography
https://scholar.google.com/citations?user=Pv1YcbgAAAAJ&hl=en
https://scholar.google.fr/citations?user=M8sxKP8AAAAJ&hl=fr
Selected publications
Molinié T, Cougouilles E, David C, Cahoreau E, Portais JC, Mourier A.
MDH2 produced OAA is a metabolic switch rewiring the fuelling of the respiratory chain and TCA cycle.
BBA Bioenergetics. 2022 Mar 1;1863(3):148532. doi: 10.1016/j.bbabio.2022.148532.
Eduardo Silva Ramos, Elisa Motori, Christian Brüser, Inge Kühl, Benedetta Ruzzenente, Jakob D. Busch, James Stewart, Johanna Kauppila, Kjell Hultenby, Dusanka Milenkovic, Stefan Jakobs, Nils-Göran Larsson★ and Arnaud Mourier★
Mitochondrial Fusion is required for regulation of mitochondrial DNA replication
PLoS Genetics Mar 2019 Jun 6;15(6):e1008085. doi: 10.1371/journal.pgen.1008085. (★co-corresponding author)
Brandt T, Mourier A, Tain LS, Partridge L, Larsson NG, Kühlbrandt W.
Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila.
Elife. 2017 Jul 12;6
Mourier A, Motori E, Brandt T, Lagouge M, Atanassov I, Galinier A, Rappl G, Brodesser S, Hultenby K, Dieterich C, Larsson NG.
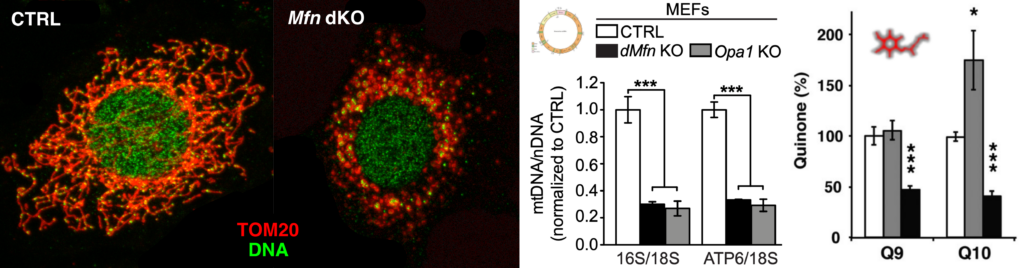
Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels.
J Cell Biol. 2015 Feb 16;208(4):429-42
Arnaud Mourier, Stanka Matic, Benedetta Ruzzenente, Nils-Göran Larsson*, Dusanka Milenkovic.. The respiratory chain supercomplex organization is independent of COX7a2l isoforms.
Cell Metabolism. 2014 December 2, 20 :1069-1075
Financial Support